When Blotting Dry a Stained Slide What Will Happen if You Rub It From Side to Side
Overview
Source: Rhiannon M. LeVequei, Natalia Martinane, Andrew J. Van Alst1, and Victor J. DiRita1
1 Department of Microbiology and Molecular Genetics, Michigan State Academy, E Lansing, Michigan, United States of America
Bacteria are diverse microorganisms found nearly everywhere on Earth. Many properties assist distinguish them from each other, including but not limited to Gram-staining type, shape and arrangement, production of capsule, and formation of spores. To observe these properties, one can use lite microscopy; withal, some bacterial characteristics (for example size, lack of coloration, and refractive properties) brand information technology hard to distinguish bacteria solely with a light microscope (1, ii). Staining bacteria is necessary when distinguishing bacterial types with low-cal microscopy. The 2 main types of light microscopes are unproblematic and compound. The chief difference between them is the number of lenses used to magnify the object. Unproblematic microscopes (for instance a magnifying drinking glass) accept but 1 lens to magnify an object, while compound microscopes take several lenses to enhance magnification (Figure 1). Compound microscopes have an objective lens close to the object which collects light to create an image of the object. This is and so magnified by the eyepiece (ocular lens) which enlarges the image. Combining the objective lens and eyepiece allows for higher magnification than using a single lens alone. Typically, compound microscopes have multiple objective lenses of varying powers to allow for different magnification (1, 2). Hither, we will hash out visualizing bacteria with Gram stains, Capsule stains, and Endospore stains.
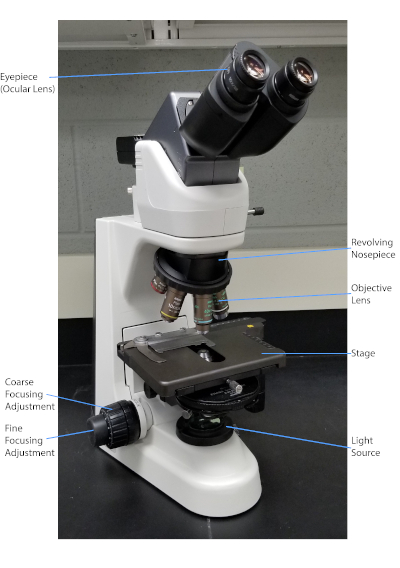
Figure 1: A typical compound microscope. The nigh important parts of the microscope are labeled.
The Gram stain, developed in 1884 by the Danish bacteriologist Hans Christian Gram (1), differentiates bacteria based on the composition of the cell wall (one, 2, 3, 4). Briefly, a bacterial smear is placed on a microscope slide and then heat-fixed to adhere the cells to the slide and make them more readily accepting of stains (1). The heat-fixed sample is stained with Crystal Violet, turning the cells purple. The slide is flushed with an Iodine solution, which fixes the Crystal Violet to the cell wall, followed past a decolorizer (an alcohol) to wash abroad whatsoever non-stock-still Crystal Violet. In the final step, a counterstain, Safranin, is added to colour cells red (Figure two). Gram-positive bacteria stain royal due to the thick peptidoglycan layer which is not easily penetrated by the decolorizer; Gram-negative bacteria, with their thinner peptidoglycan layer and higher lipid content, destain with the decolorizer and are counterstained ruddy when Safranin is added (Figure 3). Gram staining is used to differentiate cells into 2 types (Gram-positive and Gram-negative) and is likewise useful to distinguish jail cell shape (spheres or cocci, rods, curved rods, and spirals) and system (single cells, pairs, bondage, groups, and clusters) (1, 3).
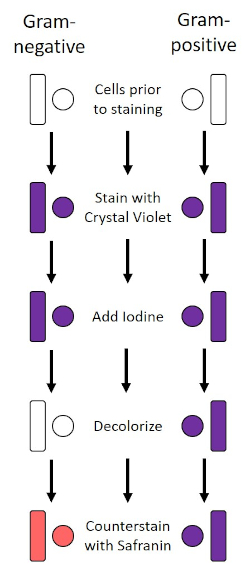
Figure 2: Schematic of the Gram Staining Protocol. The left column shows how Gram-negative bacteria react at each step of the protocol. The correct column shows how Gram-positive bacteria react. Also, shown are two typical bacterial jail cell shapes: the bacilli (or rods) and the cocci (or spheres).
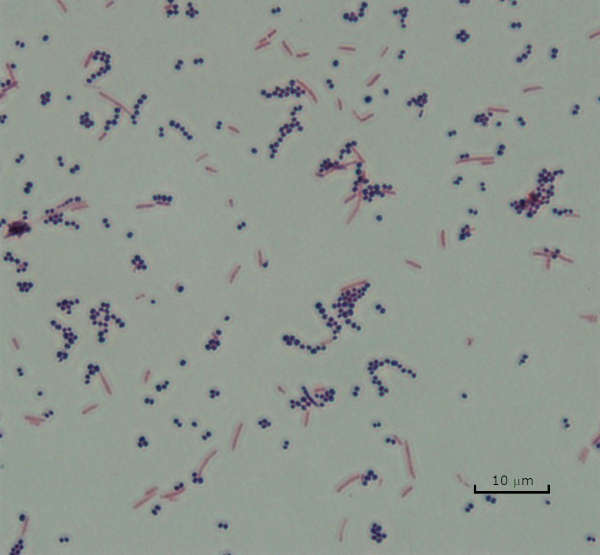
Figure three: Gram Staining Results. A Gram stain of a mixture of Staphylococcus aureus (Gram-positive purple cocci) and Escherichia coli (Gram-negative red rods).
Some bacteria produce an extracellular gluey outer layer chosen a sheathing (3, 5). Capsules are protective structures with various functions, including but not limited to adherence to surfaces and other bacteria, protection from desiccation, and protection from phagocytosis. Capsules are typically composed of polysaccharides containing more than 95% h2o, just some may contain polyalcohols and polyamines (5). Due to their mostly non-ionic composition and tendency to repel stains, simple staining methods practise not piece of work with capsule; instead, capsule staining uses a negative staining technique which stains the cells and the background, leaving the capsule as a articulate halo effectually the cells (1, three) (Figure 4). Sheathing staining involves smearing a bacterial sample into an acidic stain on a microscope slide. Different Gram staining, the bacterial smear is not rut-fixed during a Capsule Stain. Rut-fixing tin can disrupt or dehydrate the sheathing, leading to false negatives (5). Furthermore, estrus-fixing can shrink cells resulting in a immigration around the cell which can be mistaken as a capsule, leading to fake positives (3). The acidic stain colors the slide background; while follow up with a bones stain, Crystal Violet, colors the bacterial cells themselves, leaving the capsule unstained and appearing as a clear halo between the cells and the slide background (Figure 5). Traditionally, India ink has been used equally the acidic stain because these particles cannot penetrate the capsule. Therefore, neither the capsule nor the prison cell is stained by India ink; instead, the background is stained. Congo Red, Nigrosin, or Eosin tin be used in identify of India ink. Capsule staining can assist doctors diagnose bacterial infections when looking at cultures from patient samples and guide appropriate patient treatment. Common diseases caused by encapsulated leaner include pneumonia, meningitis, and salmonellosis.
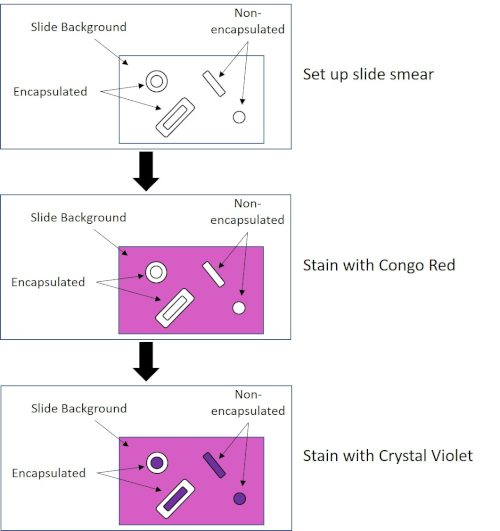
Figure 4: Schematic of the Capsule Staining Protocol. The top panel shows the slide smear prior to any stain application. The middle console shows how the slide and leaner wait after the principal stain, Congo Red. The final console shows how the slide and leaner look after the counterstain, Crystal Violet.
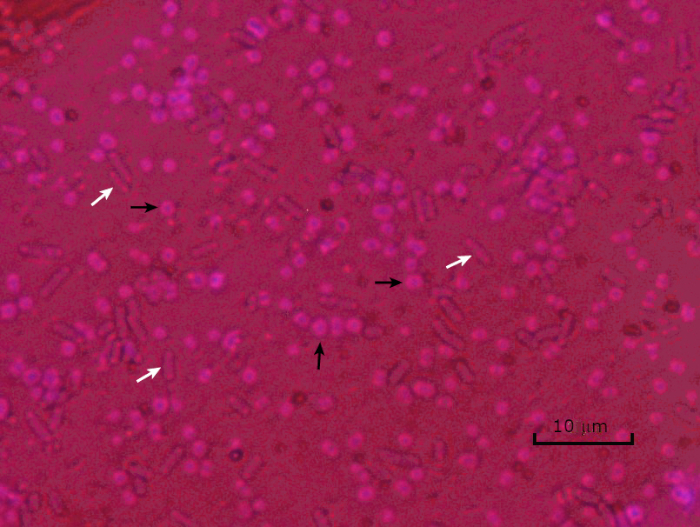
Figure 5: Capsule Staining Results. Capsule staining of encapsulated Acinetobacter baumannii (denoted with black arrows) and non-encapsulated Escherichia coli (denoted with white arrows). Detect the background is dark and A. baumannii cells are stained imperial. The sheathing around A. baumannii cells is evident equally a halo, while E. coli has no halo.
In adverse conditions (for example nutrient limitation, extreme temperatures, or dehydration), some bacteria produce endospores, metabolically inactive structures that are resistant to physical and chemical impairment (1, two, 8, 9). Endospores let the bacterium to survive harsh conditions by protecting the genetic textile of the cells; once conditions are favorable for growth, spores germinate, and bacterial growth continues. Endospores are difficult to stain with standard staining techniques because they are impermeable to dyes typically used for staining (1, 9). The technique routinely used to stain endospores is the Schaeffer-Fulton Method (Figure 6), which uses the primary stain Malachite Green, a water soluble stain that binds relatively weakly to cellular material, and rut, to permit the stain to break through the cortex of the spore (Figure 7). These steps color the growing cells (termed vegetative cells in the context of endospore biology), likewise as endospores and whatever gratuitous spores (those no longer inside the quondam cell envelope). Vegetative cells are washed with h2o to remove Malachite Green; endospores retain the stain due to heating the Malachite Dark-green within the spore. Finally, the vegetative cells are counterstained with Safranin to visualize (Figure viii). Staining for endospores helps differentiate bacteria into spore formers and non-spore formers, every bit well as determines whether spores are present in a sample which, if present, could lead to bacterial contamination upon formation.
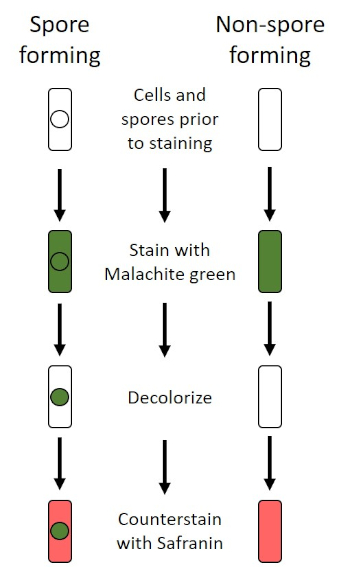
Figure 6: Schematic of the Endospore Staining Protocol. The left column shows how spore forming bacteria react at each step of the protocol. The right column shows how not-spore forming leaner react.
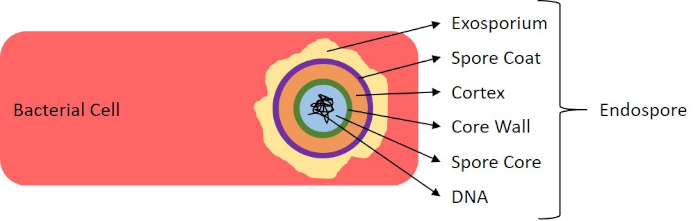
Figure 7: Diagram of Endospore Structure. Bacterial cell containing an endospore with the diverse spore structures labeled.
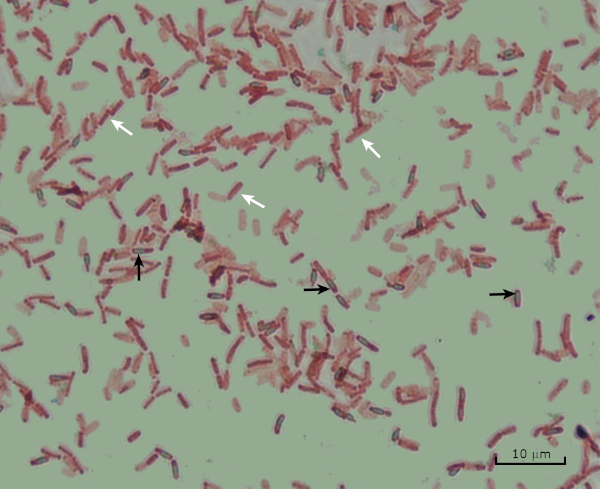
Figure 8: Endospore Staining Results. A typical staining of endospores of Bacillus subtilis. The vegetative cells (denoted with the white arrows) are stained carmine, while the endospores (denoted with the blackness arrows) are stained green.
Procedure
Log in or Outset trial to admission full content. Learn more than about your institution's access to JoVE content here
1. Gram Staining
- Fix-up
- Wear gloves and a non-flammable lab coat, as dyes will stain easily and clothing.
- A Bunsen burner is used to oestrus-prepare the bacteria. Utilize care when working with flame; tie back long pilus.
- Commercially available Gram stain reagents will be used.
- Make clean microscope slides with laboratory wipes.
- Protocol
- Pipet x µL phosphate-buffered saline or culture goop onto the slide.
- Smear a bacterial colony into the liquid to produce a thin, even layer.
Note: Exercise non use cultures older than 24 hours, as bacteria as well old could have changes in their cell wall, which will affect the Gram stain results (1, four). - Completely air-dry out slide.
- Once dried, heat-set bacteria by passing slide through the flame (bacteria side up) 4-5 times.
Note: Do not hold the slide in the flame besides long or yous might distort the bacterial cells (1). - Working over the sink, concord the slide level and apply Gram'southward Crystal Violet to completely cover the heat-fixed bacteria, allow to stand 45 seconds.
- Rinse backlog Crystal Violet past belongings the slide at an angle and squirting a gentle, indirect stream of water onto the slide and letting it run down over the stained leaner. Exercise not eject water direct onto the bacteria.
- Holding slide level again, apply Gram's Iodine Solution to completely cover the stained bacteria, allow to stand 45 seconds.
- Rinse excess Iodine every bit in pace 1.2.6 higher up.
- While holding the slide at an angle, add together a few drops of Decolorizer onto the slide, letting it trickle down over the stained leaner merely until the runoff is clear; typically, most 5 seconds. Immediately rinse with water every bit in step 1.ii.6 above.
Note: This is a disquisitional stride in the protocol. Allowing Decolorizer to trickle too long or non long plenty will result in false Gram-staining (4). - Holding the slide level once again, utilize Gram's Safranin to completely cover the bacteria, permit to stand 45 seconds.
- Rinse excess Safranin as in step one.2.6 to a higher place.
- Absorb, do not rub, excess water from slide using paper towels.
- Examine slide on the microscope using oil-immersion with a 100X objective.
- Results and Data Analysis
- Gram-positive bacteria volition stain purple.
- Gram-negative leaner will stain cherry.
- Shape (cocci, bacilli, curved rods, spirals) of bacteria will exist visible.
- System of bacterial cells (single cells, paired cells, chains of cells, clusters, groupings) volition be visible.
ii. Sheathing Staining
- Set-up
- Wear gloves and a lab glaze as dyes stain hands and clothing.
- To fix one% Crystal Violet solution, mix 0.25 gram Crystal Violet with 25 mL distilled h2o until dissolved.
- To fix 1% Congo Red solution, mix 0.25 gram Congo Crimson with 25 mL distilled water until dissolved.
- Clean slides with laboratory wipes.
- Protocol
- Place 10 µL Congo Red on slide.
- Using a pipet tip, smear a bacterial colony into the dye to produce a thin even layer.
- Completely air-dry slide with dye/cell mixture, five-seven minutes.
Notation: Do not oestrus set as heating tin dehydrate or distort the capsule. - Alluvion the smear with 1% Crystal Violet for one infinitesimal.
- Rinse backlog stain past holding the slide at an bending and squirting a gentle, indirect stream of h2o onto the slide and letting it run down over the stained leaner. Do not squirt h2o directly onto the bacteria.
- Hold the slide at a 45-degree bending until completely air-dried.
- Examine smear on the microscope under oil immersion with a 100X objective.
- Results and Information Analysis
- Bacterial cells volition stain imperial.
- The background of the slide volition stain dark.
- Capsules will be a articulate halo around cells against a nighttime groundwork.
3. Endospore Staining (Schaeffer-Fulton method)
- Fix-up
- Wear gloves and a not-combustible lab coat to protect hands and clothing from dyes and flame.
- A Bunsen burner is used to heat ready the bacteria. Utilise intendance when working with flame; tie back long pilus.
- To prepare 0.5% Malachite Green solution, mix 0.125 grams Malachite Dark-green with 25 mL distilled h2o until dissolved.
- Utilize commercially available Gram's Safranin reagent solution.
- Clean slides with laboratory wipes.
- Protocol
- Pipet ten µl phosphate buffered saline (PBS) or civilization broth onto slide.
- Using hygienic technique, smear a bacterial colony into the liquid to produce a thin, fifty-fifty layer.
Note: Endospores mostly do non grade in young cells; therefore, the culture is recommended to be between 18 and 36 hours old (9). - Completely air-dry slide.
- Oestrus gear up by passing slide (bacteria side up) through flame 4-five times.
- To help contain the dye, place a slice of lens paper (cut to fit the bacterial smear) over the estrus-fixed smear.
- Saturate lens paper with Malachite Light-green solution.
- Place slide on top of the beaker of humid water on a hot plate, and steam slide for 5 minutes, keeping lens paper moist by adding more than dye a drop at a time as needed.
Note: Avoid overheating and drying out the dye solution. - Remove slide from chalice, remove and discard lens paper, allow slide to cool ii minutes.
- Belongings slide at an angle, rinse thoroughly by squirting a gentle, indirect stream of water onto slide, allowing it to bleed down over smear.
- Holding slide level, overflowing smear with Safranin, allow to stand up one minute.
- Rinse backlog Safranin as in step 3.ii.9 above.
- Allow to air-dry.
- Examine slide on microscope nether oil-immersion with a 100X objective.
- Results and Information Analysis
- Spores will stain light-green.
- Vegetative cells volition stain cherry.
- Some vegetative cells will contain spores; the cells volition stain red, while the endospores will stain green.
-Leaner are microscopic living organisms that have many distinguishing characteristics such as shape, arrangement of cells, whether or not they produce capsules, and if they form spores. These features tin can all be visualized by staining and help in the identification and classification of unlike bacterial species.
To examine the kickoff two characteristics of jail cell shape and arrangement, we can use a uncomplicated technique called Gram staining. Hither, crystal violet is applied to bacteria, which have been heat-fixed onto a slide. Next, Gram's iodine solution is added to the slide, resulting in the formation of an insoluble circuitous between the crystal violet and the Gram'due south iodine solution. A decolorizer is then practical and whatsoever bacteria with a thick peptidoglycan layer will stain purple, equally this layer is not hands penetrated by the decolorizer. These leaner are referred to as Gram-positive.
Gram-negative bacteria accept a thinner peptidoglycan layer and volition de-stain the decolorizer, losing the purple colour. Yet, they volition stain reddish-pink when a safranin counterstain is added, which binds to a lipopolysaccharide layer on their exterior. Once stained, the cells tin can exist observed for morphology, size, and arrangement, such as in chains or clusters, which further aids in nomenclature and identification.
Some other useful technique in the microbiologist'south toolkit is the sheathing stain, used to visualize external capsules that surround some types of bacterial cells. Due to the capsule's non-ionic composition and tendency to repel stains, simple staining methods won't work. Instead, a negative staining technique is used, which first stains the groundwork with an acidic colorant, such as Congo red, before the bacterial cells are stained with crystal violet. This leaves any capsule present as a clear halo around the cells.
The final major staining technique covered here can assist make up one's mind if the bacteria being studied forms spores. In agin conditions, some bacteria produce endospores, fallow, tough, not-reproductive structures whose master function is to ensure the survival of bacteria through periods of environmental stress, like extreme temperatures or dehydration. All the same, not all bacterial species brand endospores, and they are difficult to stain with standard techniques because they are impermeable to many dyes. The Schaeffer-Fulton method uses malachite green stain, which is applied to the leaner stock-still to a slide. The slide is then washed with water before being counterstained with Safranin. Vegetative cells will announced pink-red, while any endospores present will appear green. In this video, you volition acquire how to perform these mutual bacterial staining techniques and then examine the staining samples using calorie-free microscopy.
To begin the procedure, tie back long hair and put on the appropriate personal protective equipment, including a lab coat and gloves.
And then, clean a fresh microscope slide with a laboratory wipe. Side by side, pipette x microliters of 1X phosphate-buffered saline onto the first slide. Then, use a sterile pipette tip to select a unmarried bacterial colony from the LB agar plate. Smear the bacterial colony in the liquid to produce a thin, even layer. Prepare the slide on the benchtop, and let it to fully air dry.
In one case dried, light a Bunsen burner to heat-fix the bacteria. Using tongs, pass the slide through the burner flame several times, with the bacteria side up, taking care non to hold the slide in the flame likewise long, which may distort the cells.
Now, working over the sink, hold the slide level and apply several drops of Gram's crystal violet to completely encompass the bacterial smear and then place the slide onto the bench to stand for 45 seconds. Next, hold the slide at an angle and gently squirt a stream of water onto the pinnacle of the slide, taking care not to squirt the bacterial smear straight. Now, belongings the slide level again, apply Gram's iodine solution to completely cover the stained bacteria and then allow information technology to stand for another 45 seconds. Side by side, carefully rinse the iodine from the slide, as shown previously. While property the slide at an angle, add a few drops of Gram'southward decolorizer to the slide, allowing it to run downward over the stained bacteria, just until the run-off is clear, for approximately 5 seconds. Immediately, rinse with h2o as shown previously. This will limit over-decolorizing the smear. Next, holding the slide level over again, employ Gram's safranin counterstain to completely cover the stained bacteria. After 45 seconds, gently rinse the Safranin from the slide with water, as shown previously, and then blot dry out with newspaper towels.
Finally, add a drop of immersion oil directly to the slide, and then examine the slide using a lite microscope with a 100X oil objective lens.
To begin this staining protocol, first put on the correct personal protective equipment and then ensure that the drinking glass slides that will be used are make clean.
Next, gear up the solutions. To make 1% crystal violet solution, mix 0.25 grams of crystal violet powder with 25 milliliters of distilled water and vortex until dissolved. And then, ready i% Congo blood-red solution by mixing 0.25 grams of Congo ruby-red pulverisation with 25 milliliters of distilled h2o and vortex until dissolved. Now, pipette ten microliters of the Congo ruby-red solution onto the slide. Using a clean, sterile pipette tip, select a unmarried bacterial colony from the LB agar plate. Then, smear the bacterial colony into the dye to produce a thin, even layer. Completely air dry the bacterial slide for 5-7 minutes. Once the slide is dry, flood the smear with plenty i% crystal violet to cover the smear and let it sit for 1 infinitesimal. Now, hold the slide at an angle and gently squirt a stream of water onto the top of the slide, taking intendance non to squirt the bacteria directly. Keep holding the slide at a 45-degree angle until completely air-stale. Finally, add a drop of immersion oil directly to the slide, and and so examine the slide using a light microscope with a 100X oil objective.
To perform endospore staining, first, set a 0.five% malachite light-green solution by mixing 0. 125 grams of malachite light-green powder with 25 milliliters of distilled water, and then vortex the solution until dissolved. Next, pipette ten microliters of 1X PBS onto the center of the slide. And then, apply a sterile pipette tip to select a unmarried bacterial colony from the LB agar plate. Smear the bacteria into the liquid to produce a thin, even layer. Now, prepare the slide on the benchtop, and allow it to fully air dry out. Once dried, light a Bunsen burner to rut-fix the bacteria. Pass the slide through the bluish burner flame several times, with the bacteria side facing up. And then, once the slide has cooled, place a piece of precut lens paper over the rut-fixed smear. Next, turn on a hotplate to the highest setting, and bring a beaker of water to a boil.
Saturate the lens paper with the malachite green solution and, using tongs, place the slide on meridian of the beaker of boiling water to steam for 5 minutes. Go on the lens paper moist by adding more dye, one drop at a time, as needed. Next, again using tongs, pick upward the slide from the chalice and remove and discard the lens newspaper. Allow the slide to absurd for 2 minutes. Working over the sink, hold the slide at an angle, and gently eject a stream of h2o onto the top of the slide. Now, hold the slide level and apply Safranin to completely cover the slide. Then, allow it to stand for one infinitesimal. Adjacent, hold the slide at an angle and rinse as previously shown. Let the slide to air dry out on the benchtop. Finally, add a drop of immersion oil directly to the slide, and then examine the slide with a light microscope, with a 100X oil objective.
In the Gram staining protocol, two different colored stains can result. Night purple staining indicates that the bacteria are Gram-positive and that they accept retained the crystal violet stain. In contrast, ruby-red-pink staining is a characteristic of Gram-negative bacteria, which instead volition be colored by the Safranin counterstain. Additionally, different shapes and arrangements of leaner can be visualized later Gram staining. For instance, information technology is possible to differentiate Cocci, or round bacteria, from rod-shaped Bacillus, or place leaner, which forms strands, compared to those which typically aggregate as clumps or occur singly.
In a capsule stained microscope image, the bacterial cells will typically be stained royal, and the background of the slide should be darkly stained. Against this dark groundwork, the capsules of the bacteria, if present, will announced as a clear halo around the cells.
Lastly, in endospore staining, Vegetative cells will be stained red by the Safranin counterstain. If endospores are present in the sample, these will retain the malachite light-green stain and appear bluish-dark-green in color.
Subscription Required. Please recommend JoVE to your librarian.
Applications and Summary
Log in or Start trial to admission total content. Learn more well-nigh your institution'south access to JoVE content hither
Bacteria take distinguishing characteristics that tin aid in their identification. Some of these characteristics can be observed by staining and light microscopy. 3 staining techniques useful for observing these characteristics are Gram staining, Capsule staining, and Endospore staining. Each technique identifies different characteristics of bacteria and can be used to help physicians recommend treatments for patients, place potential contaminants in samples or food products, and verify sample sterility.
Subscription Required. Please recommend JoVE to your librarian.
References
- Blackness, J. G. Microbiology Principles and Explorations, 4th edition. Prentice-Hall, Inc., Upper Saddle River, New Jersey. (1999)
- Madigan, 1000. T. and J. M. Martinko. Brock Biology of Microorganisms, xith edition. Pearson Prentice Hall, Upper Saddle River, New Jersey. (2006).
- Leboffe, One thousand. J., and B. E. Pierce. A Photographic Atlas for the Microbiology Laboratory, second ed. Morton Publishing Company, Englewood, Colorado. (1996).
- Smith, A. C. and Grand. A. Hussey. Gram stain protocols. Laboratory Protocols. American Society for Microbiology, Washington, DC. Available from: http://www.asmscience.org/content/education/protocol/protocol.2886. (2005).
- Hughes, R. B. and A. C. Smith. Capsule Stain Protocols Laboratory Protocols. American Guild for Microbiology, Washington, DC. Available from: http://www.asmscience.org/content/teaching/protocol/protocol.3041. (2007).
- Anthony, E. E. Jr. A annotation on sheathing staining. Science 73(1890):319-320 (1931).
- Finegold, Southward. 1000., W. J. Martin, and E. Yard. Scott. Bailey and Scott'south Diagnostic Microbiology, 5th edition. The C. V. Mosby Company, St. Louis, Missouri. (1978).
- Gerhardt, P., R. G. East. Murray, Due west. A. Woods, and N. R. Krieg. Methods for general and molecular bacteriology. ASM Press, Washington, DC. (1994).
- Hussey, Thou. A. and A. Zayaitz. Endospore Stain Protocol. Laboratory Protocols. American Society for Microbiology, Washington, DC. Bachelor from: http://www.asmscience.org/content/pedagogy/protocol/protocol.3112. (2007).
Bacteria are microscopic living organisms that have many distinguishing characteristics such equally shape, organisation of cells, whether or not they produce capsules, and if they grade spores. These features tin can all exist visualized by staining and aid in the identification and classification of different bacterial species.
To examine the first two characteristics of cell shape and arrangement, we tin can apply a unproblematic technique called Gram staining. Hither, crystal violet is applied to bacteria, which have been estrus-stock-still onto a slide. Next, Gram'southward iodine solution is added to the slide, resulting in the formation of an insoluble complex betwixt the crystal violet and the Gram's iodine solution. A decolorizer is then applied and any bacteria with a thick peptidoglycan layer will stain purple, every bit this layer is non easily penetrated past the decolorizer. These bacteria are referred to as Gram-positive.
Gram-negative bacteria accept a thinner peptidoglycan layer and will de-stain the decolorizer, losing the purple colour. However, they will stain reddish-pink when a safranin counterstain is added, which binds to a lipopolysaccharide layer on their outside. Once stained, the cells can be observed for morphology, size, and organisation, such as in chains or clusters, which further aids in classification and identification.
Some other useful technique in the microbiologist'due south toolkit is the capsule stain, used to visualize external capsules that surround some types of bacterial cells. Due to the capsule's not-ionic limerick and tendency to repel stains, simple staining methods won't work. Instead, a negative staining technique is used, which start stains the background with an acidic colorant, such as Congo cherry-red, before the bacterial cells are stained with crystal violet. This leaves whatsoever sheathing present as a articulate halo around the cells.
The final major staining technique covered here tin can assist determine if the bacteria being studied forms spores. In adverse weather condition, some bacteria produce endospores, fallow, tough, non-reproductive structures whose main function is to ensure the survival of bacteria through periods of environmental stress, like extreme temperatures or dehydration. However, non all bacterial species make endospores, and they are difficult to stain with standard techniques because they are impermeable to many dyes. The Schaeffer-Fulton method uses malachite green stain, which is applied to the bacteria stock-still to a slide. The slide is and then done with water earlier being counterstained with Safranin. Vegetative cells will announced pinkish-red, while any endospores present will appear green. In this video, you volition learn how to perform these common bacterial staining techniques and so examine the staining samples using light microscopy.
To begin the procedure, tie back long pilus and put on the appropriate personal protective equipment, including a lab coat and gloves.
So, clean a fresh microscope slide with a laboratory wipe. Next, pipette 10 microliters of 1X phosphate-buffered saline onto the get-go slide. Then, use a sterile pipette tip to select a unmarried bacterial colony from the LB agar plate. Smear the bacterial colony in the liquid to produce a thin, even layer. Prepare the slide on the benchtop, and allow information technology to fully air dry.
Once dried, light a Bunsen burner to heat-fix the bacteria. Using tongs, pass the slide through the burner flame several times, with the bacteria side up, taking care non to concur the slide in the flame as well long, which may distort the cells.
Now, working over the sink, agree the slide level and utilise several drops of Gram's crystal violet to completely embrace the bacterial smear and then identify the slide onto the bench to stand up for 45 seconds. Next, hold the slide at an angle and gently squirt a stream of h2o onto the superlative of the slide, taking intendance not to eject the bacterial smear directly. Now, belongings the slide level again, apply Gram's iodine solution to completely cover the stained bacteria and then allow information technology to stand up for some other 45 seconds. Next, carefully rinse the iodine from the slide, as shown previously. While holding the slide at an angle, add a few drops of Gram's decolorizer to the slide, allowing it to run down over the stained leaner, merely until the run-off is clear, for approximately 5 seconds. Immediately, rinse with water as shown previously. This will limit over-decolorizing the smear. Next, holding the slide level again, apply Gram's safranin counterstain to completely cover the stained bacteria. After 45 seconds, gently rinse the Safranin from the slide with water, as shown previously, and then blot dry out with paper towels.
Finally, add a drib of immersion oil directly to the slide, and then examine the slide using a light microscope with a 100X oil objective lens.
To brainstorm this staining protocol, get-go put on the correct personal protective equipment and then ensure that the glass slides that volition exist used are clean.
Adjacent, prepare the solutions. To make 1% crystal violet solution, mix 0.25 grams of crystal violet powder with 25 milliliters of distilled h2o and vortex until dissolved. Then, prepare ane% Congo red solution by mixing 0.25 grams of Congo ruby-red powder with 25 milliliters of distilled water and vortex until dissolved. Now, pipette 10 microliters of the Congo blood-red solution onto the slide. Using a make clean, sterile pipette tip, select a single bacterial colony from the LB agar plate. Then, smear the bacterial colony into the dye to produce a thin, even layer. Completely air dry the bacterial slide for 5-7 minutes. Once the slide is dry, flood the smear with enough 1% crystal violet to cover the smear and permit it sit for 1 infinitesimal. Now, hold the slide at an angle and gently squirt a stream of water onto the top of the slide, taking care not to eject the leaner directly. Go on holding the slide at a 45-degree angle until completely air-dried. Finally, add a drib of immersion oil directly to the slide, and so examine the slide using a light microscope with a 100X oil objective.
To perform endospore staining, first, prepare a 0.five% malachite green solution by mixing 0. 125 grams of malachite greenish powder with 25 milliliters of distilled water, and then vortex the solution until dissolved. Adjacent, pipette ten microliters of 1X PBS onto the center of the slide. Then, use a sterile pipette tip to select a single bacterial colony from the LB agar plate. Smear the leaner into the liquid to produce a thin, even layer. Now, ready the slide on the benchtop, and allow it to fully air dry. One time dried, light a Bunsen burner to rut-fix the bacteria. Pass the slide through the blue burner flame several times, with the bacteria side facing upwards. So, once the slide has cooled, identify a piece of precut lens paper over the rut-fixed smear. Adjacent, turn on a hotplate to the highest setting, and bring a beaker of h2o to a boil.
Saturate the lens newspaper with the malachite greenish solution and, using tongs, place the slide on height of the beaker of humid water to steam for 5 minutes. Keep the lens paper moist by adding more dye, one drib at a time, every bit needed. Next, once again using tongs, pick up the slide from the beaker and remove and discard the lens paper. Let the slide to cool for 2 minutes. Working over the sink, hold the slide at an angle, and gently eject a stream of water onto the top of the slide. Now, concord the slide level and apply Safranin to completely cover the slide. Then, allow it to correspond 1 minute. Next, hold the slide at an angle and rinse every bit previously shown. Allow the slide to air dry on the benchtop. Finally, add a drop of immersion oil direct to the slide, and and then examine the slide with a light microscope, with a 100X oil objective.
In the Gram staining protocol, ii different colored stains can result. Dark purple staining indicates that the bacteria are Gram-positive and that they take retained the crystal violet stain. In contrast, reddish-pink staining is a characteristic of Gram-negative bacteria, which instead will exist colored by the Safranin counterstain. Additionally, unlike shapes and arrangements of bacteria can be visualized after Gram staining. For example, information technology is possible to differentiate Cocci, or round bacteria, from rod-shaped Bacillus, or place bacteria, which forms strands, compared to those which typically aggregate as clumps or occur singly.
In a capsule stained microscope prototype, the bacterial cells will typically be stained purple, and the background of the slide should be darkly stained. Against this night background, the capsules of the leaner, if present, will appear equally a clear halo around the cells.
Lastly, in endospore staining, Vegetative cells will be stained ruddy by the Safranin counterstain. If endospores are present in the sample, these will retain the malachite green stain and announced bluish-greenish in color.
Source: https://www.jove.com/v/10513/microscopy-and-staining-gram-capsule-and-endospore-staining
Post a Comment for "When Blotting Dry a Stained Slide What Will Happen if You Rub It From Side to Side"